Background: We started a randomized phase II trial [NCT01572662] that compared the safety of two myeloablative fractionated ("timed-sequential") busulfan with fludarabine (Bu-Flu) conditioning regimens: one with a lower dose of busulfan (area under the curve [AUC] of 16 000 μmol.min; 16K arm) and one with a higher dose (AUC of 20 000 μmol.min; 20K arm). After 49 patients were treated on the 16K group and 48 patients on the 20K group, the randomization was stopped as the higher dose arm was found to be as safe as the lower dose arm. The outcomes of those patients were previously reported, with the primary endpoint of interest being day 100 non-relapse mortality (NRM). The trial then continued enrolment as a single-arm study with increased accrual onto the higher dose arm. The current paper reports long-term outcomes of a total of 150 patients treated on the higher dose arm with an extended median follow-up of over 3.5 years.
Methods: Patients with hematological malignancies up to 75 years of age were included. Bu dosing was determined on the basis of pharmacokinetic (PK) analyses conducted after day -13 and day -6 dose to achieve target AUC 20 000 ± 12% μmol.min (20K arm). On days −13 and −12, patients received 80 mg/m2 Bu IV daily as outpatient. Then, Flu 40 mg/m2 and Bu IV once daily were given as inpatient from day −6 though −3. Graft-versus-host disease (GVHD) prophylaxis consisted of tacrolimus from day −2 and methotrexate on days 1, 3, 6, and 11.
Results: The median age was 61 years (interquartile range, 55-67); most were males (91; 61%) had an unrelated donor (n=93, 62%) and received peripheral blood graft (n=110, 73.3%). The most common diagnoses were acute myeloid leukemia (AML) and myelodysplastic syndrome (n = 88, 58.7%). Among AML, 41% (n=24) were in CR, 44% (n=26) had primary induction failure and 15% (n=9) had relapsed disease without attaining CR before HCT. Over half had HCT-Specific Comorbidity Index (HCT-CI) >3 (n=79, 52.7%).
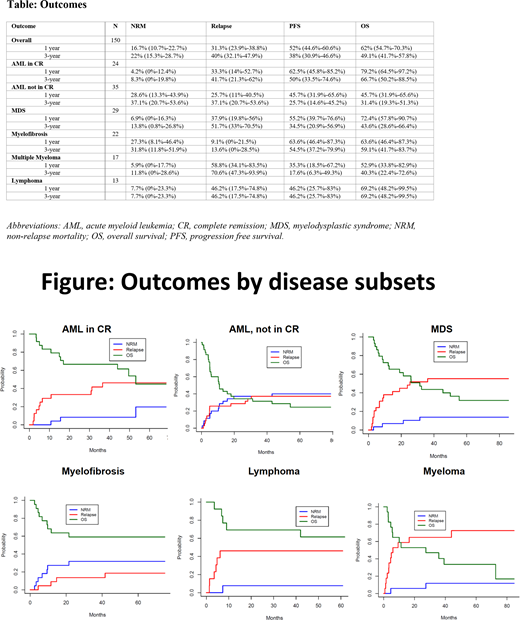
Estimated relapse, NRM, and overall survival (OS) were 40% (95% confidence interval (CI), 32.1%-47.9%), 22% (95% CI, 15.3%-28.7%), and 49.1% (95% CI, 41.7%-57.8%) at 3 years [Table]. The highest relapse rate at 3 years was noted in patients with myeloma (70.6%), followed by MDS (51.7%), and lymphoma (46.2%), while it was the lowest in myelofibrosis (13.6%). Among AML patients not in CR, the rate of relapse was not higher than those who were in CR (37.1% and 41.7%, respectively at 3 years). NRM at 3 years ranged from 7.7% (lymphoma) to 37.1% (AML, not in CR). Lymphoma patients had the lowest NRM (7.7%) and the best OS (69.2%) at 3 years, while AML patients not in CR had the highest NRM (37.1%) and the lowest OS (31.4%) [Figure]. Patients with HCT-CI 0-2 had lower NRM (14.1%; 95% CI, 5.9%-22.3%) and better OS (57.2%; 95% CI, 46.7%-70.1%) than those with HCT-CI > 3 (NRM: 29.1%; 95% CI, 19%-39.2% & OS: 41.7%; 95% CI, 32.2%-54.2%).
Day 100 cumulative incidence of grade II-V acute GVHD was 38% (95% CI, 30.2%-45.8%), grade III-IV was 11.3% (95% CI, 6.2%- 16.4%). At 3 years, cumulative incidence of extensive chronic GVHD was 27% (95% CI, 20%-34%), bronchiolitis obliterans was 4.7% (95% CI, 1.3%-8.1%), and secondary malignancies was 8.7% (95% CI, 4.1%-13.2%).
Conclusion: The fractionated myeloablative Bu-Flu conditioning regimen is well tolerated and leads to acceptable risk of NRM, relapse and long term survival in older patients, those with high risk disease and high comorbidities. Acknowledging the high risk study population, the long term outcomes, although acceptable, provide a framework to further improve upon. Modifications of this fractionated Bu-Flu regimen to further enhance its efficacy (with the addition of other chemotherapy agents) while reducing the toxicity and risk of NRM (with an inclusion of novel GVHD prophylaxis regimens) are currently being investigated.
Mehta:Incyte: Research Funding; Kadmon: Research Funding; CSL Behring: Research Funding. Alousi:Therakos: Research Funding; Alexion: Honoraria; Incyte: Honoraria, Research Funding. Bashir:Celgene: Research Funding; Amgen: Other: Advisory Board; KITE: Other: Advisory Board; Purdue: Other: Advisory Board; Takeda: Other: Advisory Board, Research Funding; Acrotech: Research Funding; StemLine: Research Funding. Hosing:NKARTA Inc.: Consultancy. Kebriaei:Novartis: Other: Served on advisory board; Amgen: Other: Research Support; Jazz: Consultancy; Kite: Other: Served on advisory board; Ziopharm: Other: Research Support; Pfizer: Other: Served on advisory board. Oran:Celgene: Consultancy; Arog Pharmaceuticals: Research Funding; ASTEX: Research Funding. Qazilbash:Angiocrine: Research Funding; Amgen: Research Funding; Bioclinica: Consultancy; Bioline: Research Funding; Janssen: Research Funding. Shpall:Takeda: Other: Licensing Agreement; Magenta: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Adaptimmune: Membership on an entity's Board of Directors or advisory committees; Zelluna: Membership on an entity's Board of Directors or advisory committees. Champlin:Takeda: Patents & Royalties; Johnson and Johnson: Consultancy; Actinium: Consultancy; Cytonus: Consultancy; Omeros: Consultancy; Genzyme: Speakers Bureau; DKMS America: Membership on an entity's Board of Directors or advisory committees. Popat:Bayer: Research Funding; Novartis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal